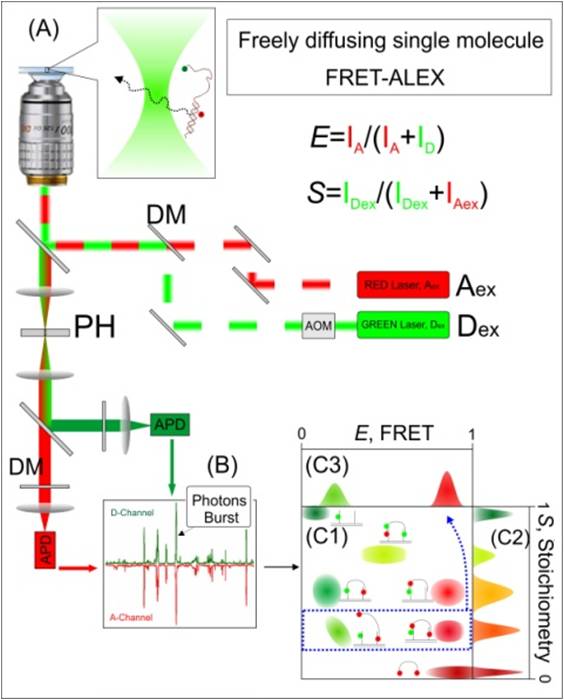
fd-sm-FRET-ALEX
Setup.
Roman
has built a setup for FRET-ALEX spectroscopy of single DNA species. In
this
setup we are able to measure picomolar concentrations of fluorescently
labeled
samples which freely-diffuse in and out of the confocal spot. Briefly, an alternating donor-excitation
laser (Dex) and an acceptor-excitation laser (Aex)
are
focused via the objective to create a diffraction limited spot (A). The
donor
dye absorbs a photon that originated from the Dex laser and
either
emits a photon or transfers the energy to the acceptor dye, which, in
turn,
emits a photon. Alternatively, the acceptor dye directly absorbs a
photon that
originated from the Aex laser and then emits a photon. The
emitted
photons are collected by the objective, split based on their
wavelengths, and
detected by a single photon detectors (APD). (B) Separate bursts of
photons,
each corresponding to a single molecule event, are detected. (C)
Schematic
representation of E and S histogram. (C1) For each burst, E (energy
transfer
Efficiency) and S (Stoichiometry) are calculated and the results are
placed in
an E/S 2D histogram. (C2) S-projection reports on the complex
structural integrity.
(C3) E-projection, in this example, of population located between S=0.4
and
S=0.3, (corresponding to one donor and two acceptors attached to a
single
molecule).
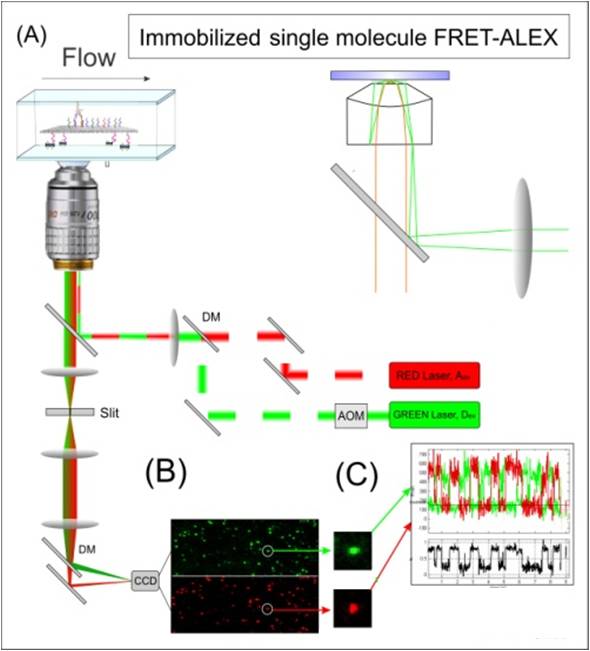
Immobilized-sm-FRET-ALEX:
(A) TIRF setup: The excitation lasers are focused on the side of the back focal-plane of a high numerical aperture objective, creating an evanescent field of ~100 nm, thereby reducing the background florescence. A low concentration of fluorescently labelled molecules is immobilized on a coverslip surface via biotin-avidin chemistry, and a flow cell allows exchanging buffer or adding of external molecules. (B) The collected photons are split to donor and acceptor channels, imaged on a fast CCD camera and recorded as a function of time. (C) Time-traces of each individual molecule are analyzed by means of FRET and ALEX.