Prof. Oren
Regev
Mesophase templated nanostructures, polymer-nanoparticles interactions.
The main emphasis of Prof. Regev research group is
on the creation of controlled nanostructures and inclusion or trapping
of active ingredients in these structures. These include mesoporous
materials with applications in chemical biochemical catalysis, interaction
between nanotubes and their surroundings and use of surface active molecules
to form solid nanowires. The most salient activities of Prof. Regev
group are briefly summarized below:
1. Gold nanoparticles integrated in onion-type
multilamellar vesicles
Gold nanoparticles have been synthesized in onion-type multilamellar
vesicles using vesicles components as the reductant. Particles size
and shape depend on whether the gold salt is introduced directly in
the vesicles or by diffusion from the external dispersion medium (see
Fig. 1). A close coupling between nanoparticle morphology and lamellar
phase is evidenced by cryo-TEM imaging.

Fig. 1: Cryo-TEM image of gold nanoparticles grown in
onions dispersed in gold salt for 12 hours. Black arrows: lamellar defects
around nanoparticles, White arrows: bending of bilayers. Bar = 200 nm.
2. Mesoporous material
A novel family of mesoporous molecular sieves (M41-S) has recently been
reported by scientists of Mobil Oil Research and Development. During
its synthesis a base- or acid-catalyzed polymerization of inorganic
compounds (silica in most cases) takes place around surfactant micelles
that serve as a templating agent. At the end of the synthesis, a silica
matrix imbedded by micelles namely, a mesoporous material, is obtained.
Its mechanism of formation is not yet completely understood. We have
studied the mechanism of formation and the phase transition sequence
occurring during the synthesis of bicontinuous cubic mesoporous phase.
It was found that the changes in the surfactant packing parameter, dictated
by the time and temperature-dependent reaction parameters, result in
a hexagonal-lamellar-hexagonal-cubic phase transition sequence. In this
study, we presented a small angle X-ray scattering (SAXS) in-situ study
of a new preparation procedure of the bicontinuous cubic (Ia3d) phase
in which an organic base is used as a reaction catalyst. The coexisting
hexagonal and lamellar phases detected at an early reaction stage could
indicate their importance in the formation of a cubic (Ia3d) mesophase.
We found that the final cubic phase is formed only when heat is applied.
The cubic phase is formed by the collapse of a preceding hexagonal phase,
where a given hexagonal plane (1 0 0) evolves to a cubic plane (2 1
1) having the same interplanar distance.
3. Conductive nanotube - polymer composites
using latex technology
Nanotubes are promising fillers for a polymer matrix and are expected
to enhance both electrical and mechanical properties of composites at
extremely low loading due to their high aspect ratio and inherent properties.
We describe experiments with composites consisting of individual, or
bundle of few single-wall, exfoliated nanotubes in a highly viscous
polymer matrix. Using latex technology we compatibilize the nanotube
and the polymer by surfactant molecules, which disperse both the nanotubes
and the latex, hence avoiding the need for direct attraction between
the nanotubes and the polymer matrix. The latter have previously limited
the spectrum of polymer type of the matrix, or required nanotube functionalization
(impairing their properties) to assure their dispersion in the matrix.
We show that a homogeneous network of nanotubes is integrated within
a polymer matrix of choice, giving rise to low resistivity with a conductivity
percolation threshold as low as 0.28 wt% nanotubes (see Fig. 2).

Fig. 2: Resistivity measurements of
composite films containing SDS-dispersed SWNT in PS (open squares) and
in PMMA (solid squares) matrix. GA-dispersed SWNT in PS (solid triangles).
PS/SDS, PMMA/SDS=30:1 w/w, PS/GA=30:5 w/w
4. Hierarchically Ordered CdS Nanowires dispersed
in Aqueous Solution
This work demonstrates the formation of cadmium sulfide (CdS) nanowires.
These nanowires are templated by mesoporous SBA-15 and their connectivity
is tuned by the number of nanoconnectors (templated by micropores) (cf.
Fig. 3). They form stable dispersions in aqueous sodium dodecyl sulfate
(SDS) solutions.

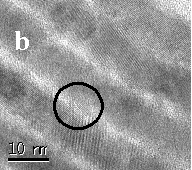
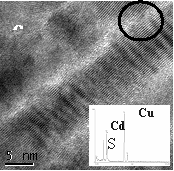
Fig 3. TEM image of silica-free CdS
nanowires calcined at 773K. The dashed zone in (a) is magnified in (b)
and further in (c). The black circles indicate the presence of nanoconnectors
between the crystalline nanowires. Inset in (c) shows EDS pattern recorded
to determine the chemical composition of the CdS nanowires

