Prof. Moshe
Gottlieb
Polymers in the vicinity of surfaces and interfaces, physical
gelation as result of microdomain confinement.
The research activity of Prof. Moshe Gottlieb's group
is related to two main topics:
(i) Polymers in the vicinity of surfaces and interfaces. This includes
the study of polymer filler interactions, effect of surface chemistry
on polymer adsorption, polymers in the vicinity of interfaces in relation
to polymeric surfactants and the effect of surfaces on polymer crystallization.
(ii) Gelation phenomena and especially physical gelation as result of
microdomain confinement.
1. Polymer-Solid interactions (Composite
Materials)
Surface Adhesion. In this area we have examined the interaction between
a polymer matrix and different solid additives used in polymer composite
materials. Although the study focused on polypropylene based matrices
the study is aimed at obtaining a more general understanding. Composite
materials demand constant improvement in mechanical properties due to
more rigorous application requirements. At the same time health, safety
and environmental requirements impose incorporation of additives which
usually affect negatively the mechanical properties of the material.
Fibers are commonly used in polymeric composites as a reinforcing agent.
The fibers are introduced to bear loads above the intrinsic mechanical
strength of the polymer matrix. A well-established interface between
the fiber and the polymer matrix will disperse the external load to
the rigid fiber and hence enhance the overall composite mechanical strength.
The results are manifested in material stiffness, toughness and even
in the failure mode of the composite.
In general, the adhesion between polymeric materials and fibers is weak
due to poor compatibility, wettability and bonding. Coupling agents
are commonly used in order to overcome the large differences between
the two components and improve their compatibility by forming better
interfacial forces ranging from strong chemical bonds or electric attraction
to weak Van der Waals (VDW) interactions. The most widely used commercial
coupling agents are silane compounds. In this work, we developed a methodology
to quantify the strength of adhesion between the polymer, (Polypropylene
(PP) or maleic anhydride grafted onto PP) and glass slabs treated by
different silane coupling agents, as a model system for glass fibers
in commercial composites.
The thickness of PP and PP-g-MA films were measured using optical phase
interference microscopy (OPIM). A scratch was made to form a clear boundary
between the polymer layer and the glass substrate, which allowed measuring
the layer thickness. Fig 1 depicts an OPIM image of a PP film deposited
on (a) untreated glass and (b) trimethoxypropylesilane (PS) treated
glass.
The PP films were found to be of similar thickness, approximately 18-20nm
irrespective of the type of the surface treatment. Scaling theory predicts
that the thickness of a polymer layer formed from melt and rinsed in
good solvent is L~aN0.83, here L~185nm. The predicted thickness is 10
times larger than the measured value. We attribute this discrepancy
to the preparation method which we speculate, leaves on the surface
only highly absorbed chains. The thickness of PP-g-MA films varied between
~10nm and 50nm depending on type of surface treatments. A more fundamental
understanding of the behavior of polymer chains in these films will
hopefully be obtained from the theoretical work of Dr. Aleksey Drozdov
(see below).

Fig 1: A. OPIM image of: PP on untreated
glass
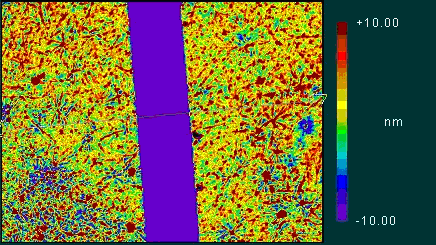
B. OPIM images of: PP on PS treated
glass
AFM was used to quantify the strength of adhesion between
the polymer and the glass surface. This was achieved by gradually increasing
the normal force applied by the AFM in 'contact mode' until reaching
the level necessary to remove completely the polymer layer. The AFM
used in non-contact 'tapping mode' was employed to determine the morphology
of the structure prior and subsequent to the polymer removal. The AFM
tapping-mode images shown in Fig. 8 depict the polymer film before and
after film removal by the contact-AFM. The measured forces for PP are
all of the same magnitude as those expected from VDW interactions and
correlate well with the nature of surface treatment: the more hydrophilic
the surface the weaker the interactions with PP. An opposite trend is
observed in the case of the PP-g-MA which suggests that the hydrophilic
MA side chains are responsible for most of the surface-polymer interaction.

Fig. 2 – Tapping mode AFM images of:
A) polymer film prior to film removal

B) After polymer film removal
Fire Retardants. Thermal polymerization of pentabromobenzylacrylate
(PBBMA) in a polypropylene (PP) composite that contains glass fibers
and magnesium hydroxide has been studied using scanning and transmission
electron microscopy techniques coupled with energy dispersive spectrometry.
The addition of PBBMA imparts flame retardant (FR) properties to the
PP composite but also affects adversely its mechanical properties. It
is of practical importance to determine the spatial distribution and
the extent of polymerization of the FR in the PP composite in order
to understand better its role in the system. The methods we have developed
allow the distinction between the monomeric and polymeric forms of the
FR and to determine their spatial distributions. In a typical PP composite
studied here we observe that the residual monomer is not dissolved in
the composite, whereas the polymeric FR is homogeneously dispersed in
PP matrix. By the combination of the electron-microscopy methods and
FTIR microscopy it was determined that the polymerization reaction is
highly hindered by the presence of the antioxidant and takes place only
in the presence of antimony oxide. PP itself shows poor adhesion to
the glass fibers (Fig. 3), which may be improved by the addition of
the reactive PBBMA. The latter is polymerized during reactive extrusion
through an antimony-catalyzed reaction. PP shows good adhesion to sized
Mg(OH)2 as expected from a properly surface-treated filler.
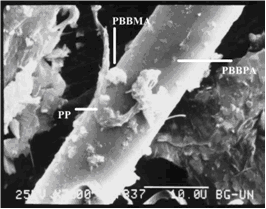
Fig 3A. Scanning electron micrograph. In this image PBBPA
spots, PBBMA particle, and a PP fibril are observed. PBBPA is detected
at the base of the fibril near the glass fiber.

Fig 3B. Scanning electron micrograph.
No PP adhesion to the glass fiber is observed. Small spots are identified
as PBBPA.
Composite Optimization. Based on the insight obtained
in the studies described above we set out to design an optimal composition
for a composite matrix containing glass fibers, PBBMA as a primary FR
and magnesium hydroxide as a secondary FR. Optimal composition is reached
by means of statistical design of experiments (DOE) rather than by ‘‘trial
and error’’ approach. The DOE approach allows minimization
of the number of experiments, investigation of the influence of each
additive and the mutual interactions between additives. It also allows
prediction of optimal sample properties with improved mechanical and
FR properties. Both FRs reduce the impact strength while enhancing flame
retardancy. Glass fibers increase the modulus, but have only a moderate
effect on the impact strength due to poor adhesion with PP. The effect
of glass fibers on the flammability of the material has still to be
understood.
Thermal Transitions. The effect of surfaces on thermal transitions in
polymeric systems has been studied experimentally and theoretically.
The enhancement of crystallization was attributed to increased ordering
of chains. The work has been described in detail (see http://echem.bgu.ac.il/staff/Rachel/index.htm).
The study of enhanced order in polymers in the vicinity of surfaces
was complemented by the study of orientation of fibers in the vicinity
of solid walls (Mor et al. J. Rheol. 2003).
2. Polymers at Interfaces
In this work we examined the properties of ABA amphiphilic triblock
copolymers at the air-liquid and liquid-liquid interfaces. The interfacial
properties of poly(ethyleneoxide) – poly(dimethylsiloxane) –
poly(ethyleneoxide) triblock copolymer (PEO-b-PDMS-b-PEO) synthesized
in our labs were investigated. Three copolymer compositions were examined
as effective surfactants. The molecular weight of the PDMS block was
12K whereas the PEO end blocks were 250, 500 and 1700 g/mol respectively.
The copolymer with the intermediate size PEO end blocks (500 g/mol)
lowered the surface tension considerably more than those with either
the smaller or larger PEO blocks. This result is in contrast with the
behavior of PEO molecules for which, the surface tension decreases as
the length of the chain increases. The observed behavior can be rationalized
by means of packing at the interface (cf. Fig. 4). The triblock copolymer
with 500 g/mol PEO block has the highest packing (~60 Å2/molecule)
at the interface. Long PEO blocks may generate a higher driving force
towards the interface, but it lacks the ability to pack densely, resulting
in fewer molecules adsorbed at the interface. From our results it appears
that there is an optimal number of EO monomers for maximum surface activity.
The optimal PEO length or PEO/PDMS ratio has not yet been determined
and, it may also vary for different liquid-liquid interfaces.

Fig 4: Estimated projected surface area at the decane-water
interface. T = 25OC
The results obtained in this study show that PEO-PDMS-PEO triblock copolymer
is highly efficient in reducing ? of the water-decane interface. The
efficiency of these materials compares very favorably with those of
other simple and polymeric surfactants (e. g. AOT, Pluronic) and was
found to be extremely effective in long-term emulsion stabilization.
The surface activity of the triblock copolymer with 11 EO monomers end-group
is considerably higher than the one with longer or shorter PEO blocks.
This behavior contradicts the behavior of the individual blocks, but
can be rationalized by means of packing at the interface. The triblock
copolymer with 11 EO monomers has the highest packing (low area per
molecule) at the interface. Longer PEO blocks may generate a higher
driving force for adsorption to the interface, but they lack the ability
to pack densely, resulting in fewer molecules adsorbing to the interface.
3. “Soft” Microdomain Interactions
This study is targeted at gels formed by a spatially-confined gelation
process. . This type of gels is of relevance to thin film technology,
membrane formation, and surface phenomena. Gelation takes place in a
spatially confined environment as result of the presence of microdomains.
The microdomains studied here are either 1) micelles formed by short
chain surfactants interacting with amphiphilic polymers or 2) microphase
separation domains as result of hydrophobic interactions. The imposed
confinement affects the dynamic properties of these systems as manifested
by the rheological properties of the resulting gels. The work involves
the study of two classes of gels: a)amphiphilic block mesogels based
on ABA copolymers of the type discussed above b)hydrophobically substituted
biopolymers (e.g. cellulose derivatives). Block copolymers are known
to form well ordered morphologies being further enhanced by the contradictory
solvation in amphiphilic systems. Such systems have potential to serve
as nanocomposite templates. Amphiphilic ABA triblock copolymer with
the different parts of the polymer residing in different solvents may
offer even a greater degree of complexity. As result of the presence
of short chain surfactants the solvent which is dispersed in the second
continuous-phase solvent may adopt micellar, cylindrical, or lamellar
mesophase morphologies. The copolymer is capable, depending on chain
and segment size and on system composition, to bridge between adjacent
dispersed aggregates. Gelation will occur when a critical bridging is
achieved resulting in the formation of a weak physical mesogels. A systematic
study of structure, morphology and rheology of the gelation process
and the resulting materials is required. Recent theoretical work by
Zilman et al. (Zilman A; Kieffer J; Molino F; Porte G; Safran SA. PRL
(2003) 91 (1): art. no.-015901) predicted a phase separated region and
two rheologically distinct phases depending on the composition of the
system. We found this description incomplete and highly dependent on
minor changes in the nature of system constituents. A typical example
of the results obtained in this ongoing study are presented in Fig.
5.

Fig. 5. Phase diagram of a system composed of isooctane/water/AOT
and ABA copolymer. The rheological behavior of a system residing in
the phase separated region indicates a gel-like behavior.
The gelation behavior of hydrohpobically modified biopolymers such as
methyl cellulose show the peculiar thermal gelation, i.e. the system
gels reversibly as result of heating. Gelation is attributed to the
formation of microphase separated domains in which the hydrophobic groups
assemble. In reversible physical gels of most natural and synthetic
polymers the process of gelation occurs upon cooling of the polymer
solution. In contrast, aqueous solutions of methylcellulose have the
unusual property of forming gels on heating and reverting to the solution
state on cooling. Gelation of methylcellulose solution is believed to
be caused primarily by the hydrophobic interaction between molecules
containing methoxyl substitution. The purpose of this paper is to investigate
the role of the chemical structure on the physical properties of methylcellulose
gels.
Two different processes for cellulose methylation are available: homogeneous
and heterogeneous. The heterogeneous process, which is carried out in
aqueous environment, is the one commonly used in industry. The cellulose
is not dissolved but rather dispersed in the medium. As result, the
methylation is believed to be unevenly distributed along the polymer
chain resulting in large hydrophobic and hydrophilic zones along the
polymer backbone. Homogeneous methylation is carried out in a solvent
capable of molecularly dissolving cellulose bundles and is expected
to yield a more uniformly distributed methylation. In this work, methylcellulose
was prepared by homogeneous reaction with different degree of substitution
(0.9<DS<2.3) and molecular weight. The structural characterization
of the samples was carried out using 13C-NMR and HPLC. Microstructures
of semi-dilute solution and gel have been tested by SAXS measurement.
Rheological studies were performed over the range temperature 25-800C
and the properties of the samples were compared with those of commercial
samples. Thermodynamic as well as kinetic effects have been examined.
Large differences were observed between the commercial (heterogeneous)
sample and the samples prepared by homogeneous reaction. To demonstrate
the highly complex morphologies involved in this process a phase diagram
complemented by cryo-TEM imaging of systems under different conditions
is presented in Fig. 6.

Fig. 6: Morphologies observed by cryo-TEM in MC solutions.
(In collaboration with Y. Talmon, Technion)

