Dr. Anne Bernheim-Groswasser
In- vitro studies of cell cytoskeleton, active polymer networks (actin)
and cell motility
Eukaryotic cells move by a complex mechanism of extension and retraction
of their actin cytoskeleton network. During locomotion, cells extend
protrusions at their leading edge, i.e., Lamellipodia and Filopodia.
Endosomes, vesicles and some pathogens move in the cell by a similar
molecular mechanism. Despite many advances in identifying the biochemical
basis of actin-dependent movement, the precise mechanism of force generation
remains unclear. The group of Dr. Bernheim-Groswasser is concerned with
elucidating the different aspects of these phenomena especially as related
to the role of biopolymers, their self aggregation, polymerization and
depolymerization, network formation and effect of surfaces as related
to cell motility and deformation. This work is carried out in a unique
laboratory dedicated for the study of biophysics of the cell cytoskeleton.
1. In vitro study of the cell cytoskeleton properties
We focused on the bulk properties of actin assembly analyzing the role
of the proteins known to control the actin cytoskeleton reorganization.
More specifically, we studied the role of Arp2/3 complex, Fascin and
capping proteins (CP) by gradually changing their ratio in the motility
medium. We show that in vitro bulk polymerization of actin in the presence
of Arp2/3 complex and fascin results in spontaneous formation of asters
(Fig. 1) and stars (Fig. 2) of actin filaments, similar actin filament
stars were recently observed in vivo [M. R. Mejillano et al. Cell 118,
363 (2004).]. Based on these observations we proposed a model for explaining
the spontaneous self-assembly of asters and stars, this model is also
relevant for understanding the transition from lamellipodia to filopodia
(Lior Haviv et al. manuscript in preparation).
Effect of surface proximity. In parallel to the work conducted in the
bulk, we started to build the experimental set-up which will be used
for studying the role of surface proximity (mimicking the role of the
cell membrane) on the system properties. For that purpose we set up
the electrofomation technique in the lab to prepare giant unilamellar
vesicles. In addition, we purified GST-WASP and GST-cdc42 proteins (plasmids
were kindly given by Prof. W. Lim, from the Dept. of the Biochemistry
and Biophysics, Univ. of California, San Franscisco).
2. Self-organization properties of active filament-motor systems:
We investigated the role of Myosin II/actin ratio on the reorganization
pathway. The role of Fascin (bundling protein) was also elaborated.
We were able to show that myosin II can reorganize actin filaments into
different microstructures, depending on myosin II concentration. Formation
of spiral, branched networks (Fig. 3) and asters (Fig. 26) were observed
experimentally. These observations are in accord with a theoretical
model published recently by our collaborators [K. Kruse et al. PRL 92,
078101 (2004)] (Prof. F. Julicher and Dr. Karsten Kruse, Max-Planck-Institute
for Physics of Complex Systems, Biological Physics Dept., Dresden).

Fig 1. Diffuse aster-like structures
are formed in bulk in the presence of Arp2/3 actin only

Fig 2. The presence of Fascin in addition
to Arp2/3 complex and actin results in the and formation of stars (bar
= 10?m)

Fig. 3. Formation of network of asters by myosin II molecular
motors

Fig. 4. Large individual asters are
also formed
3. Dynamics of active polymer networks
The study of dynamical properties of F-actin (filamentous or polymeric
form of actin) dilute solutions was conducted using FCS (fluorescence
correlation spectroscopy) technique. Using this technique we performed
non-invasive measurements of the stochastic motion of F-actin monomers
(determined by us as the elementary unit of the system, i.e., short
F-actin element) in dilute solutions [A. Bernheim-Groswasser, R. Shusterman,
and O. Krichevsky, Monomer dynamics in F-actin filaments (submitted
to Phys. Rev. Lett.)]. Fluorescent labels were placed specifically at
the ends of actin filaments. Their motion was then monitored with the
help of the FCS technique. This approach allows measuring the monomers'
mean-square displacements (MSD) as a function of time in a wide range
of timescales, from 20us to 2s (Fig.5). Over four decades in time the
monomers' MSD follows ~t3/4 power law dependence in qualitative agreement
with the current theories of semiflexible polymer dynamics. Moreover,
the experimental results agree quantitatively with the predictions of
two theories [R. Granek, J. Phys. II (France) 7, 1761 (1997); K. Kroy
and E. Frey, Phys. Rev. E. 55, 3092 (1997)], in the appropriate timescales.
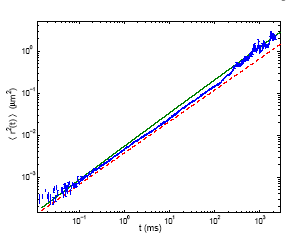
Fig 5. The kinetics of random motion
MSD <r2(t)> of actin filaments' ends as function of time.Experimental
measurements (blue points) are compared to the theoretical predictions
by Kroy and Frey (dashed red line) and Granek (solid green line).

