Dr. Amir Berman
Specific interactions at interfaces, structured interfaces, functional
Lamgmuir monolayers, biomenrals.
The group of Dr. Berman is concerned with the study
of various specific interactions at interfaces. These include structured
interfaces, Langmuir monolayers and the study of biominerals that are
the outcome of close control by specific organic macromolecules via
the organic-inorganic interface. In the case of Langmuir monolayers
special emphasis is placed on studying the structural properties and
possible applications of thin conjugated films of various polydiacetylene
(PDA) derivatives.
1. In-situ nucleation of nanoparticles on PDA.
PDA interfaces were shown to be effective templates for directed nucleation
of metal chalcogenides nanoparticles (NP) (cf. Fig. 1,2). It was found
that CdS, PbS and ZnS nucleate at the PDA-solution interface with unique
axis aligned with the PDA polymer direction. (Golan and Berman, Langmuir,
2003) The close association of the intrinsically conducting or semiconducting
polymer template and the epitaxially nucleated NP's bears the possibility
of electronic orbital overlap between the PDA and NP, thus enable the
formation of a functioning nanometer size photovoltaic elements or their
usage as light emitting objects. The PDA, being an organic, semi-rigid
template is shown to structurally respond to the underlying metal ion
in the subphase solution. This slight structural flexibility is implicated
in its efficiency as nucleating surface for many different inorganic
crystals and NP (Lifshitz, Golan, and Berman, 2004 ESRF highlights).
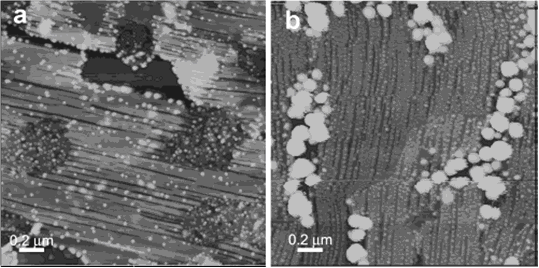
Fig. 1 AFM images of Ag2S nanocrystals on PDA. The Ag2S
nanocrystals were deposited in the presence of PDA Langmuir film from
an aqueous subphase of (a) 0.03 mM, (b)0.3 mM AgNO3.
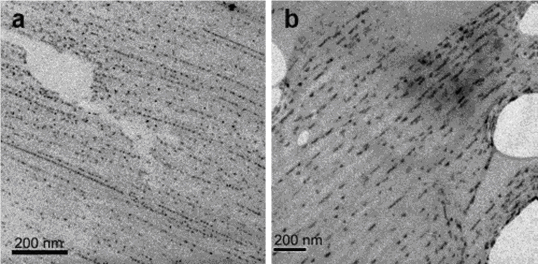
Fig. 2 TEM BF images of (a) CdS and (b) PbS nanocrystals
on PDA
2. Polydiacetylene-based Molecular Electronics
The goal of this work is to study the feasibility of using polydiacetylene
monomolecular thick aggregates for 'bottom-up' construction of functioning
nanometer-size devices, and to explore ways of combining such constructs
into circuits. While at the small scale molecular self assembly dominates,
we propose to use external forces to align such nano-constructs in the
plane. Up to this level, the assembly is done at the air-solution interface.
Investigations on the construction of the individual elements that comprise
the envisioned assembly are presently underway in different stages.
Follows are short descriptions of these projects and their possible
expected function in final assembly.
• Molecular wire. Polydiacetylene monolayers are made by application
of diacetylene containing long chain amphiphilic compounds and their
subsequent compression and UV polymerization at the air-water interface.
The resulting monolayer is highly anisotropic and organized in a striated
pattern, parallel to the polyconjugated direction.
• Control of the in-plane direction of the PDA strips. A theoretical
study indicates that the in-plane orientation of amphiphilic molecules
with an overall truncated ellipsoid cone shape is coupled with the local
surface curvature of the liquid interface. (Likhterov and Berman, submitted)
A standing wave pattern can be formed by mechanical excitation of the
liquid surface at a certain frequency. It follows that polymerizable
diacetylene molecules at the surface can be directed at will in certain
direction, and fixed at that orientation. In this scheme, the surface
wave can be excited by fabricated pattern of piezoelectric material
at the underlying surface. In-situ polymerization can also be performed
by directing UV light through fabricated waveguides.
• Integration of modifications on the wire. The electrical properties
of the conjugated system depend strongly on its conformation and the
presence of pendant electron donor or acceptor groups or its doping
the conjugated system with heavy atoms for that purpose. Several routes
are examined for the modifications of the organic molecular quasi-1-D
wire. These include derivatization of the PDA head groups with molecules
with specific recognition moieties. In particular, we derivatize diacetylene
monomers with nucleotide bases that hybridize with the complementary
oligonucleotide and deform the conjugated system in a controlled and
tunable way. (Chen and Berman, Nanotechnology, 2004) This approach provides
a means for the control of the 1-D conductor by chemical modification.
Inversely, it can be used as an integrated chemical or biosensor.
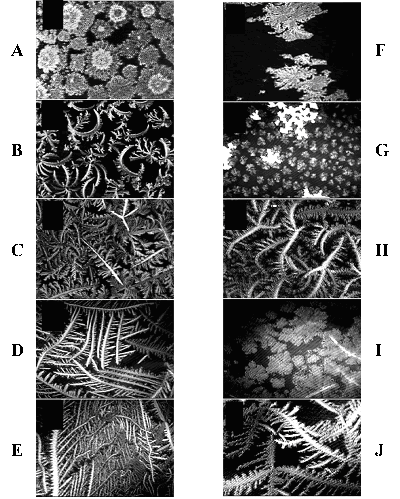
Fig. 3: BAM images of PDC/PDOH mixtures on pure buffer
((a)–(e)) and on 5 mM guanosine in Trizma buffer ((f)–(j)).
Film compositions: (a), (f) 100%; (b), (g) 90%; (c), (h) 80%; (d), (i)
70%; (e), (j) 60%. The scale is 450 µm on the long edge. Note
the disordered morphology in (a), (f), the curved morphology in (b)
and the higher curvature in (g). In (c), note the dual morphology of
the main (straight) versus the curved secondary branches. In (d) note
the long range of the undisturbed appearance. In (i), the domains exhibit
planar rather than linear appearance in the absence of guanosine (d),
suggesting enhanced lateral interactions in the presence of guanosine.
In (e) and (j), the PDC content is below the optimal 2:1 ratio, the
elongated morphology dominates and the effect of the guanosine is less
pronounced as the cytidyl moieties are more spaced.
3. Structured Interfaces
We have studied (cf. Fig. 3) the specific recognition between derivatized
PDA monolayers with nucleobases moieties and the complementary bases
and complementary short DNA oligomers, thus forming a novel biological-synthetic
macromolecular hybrid monolayer material. The underlying structural
model is aimed to demonstrate the possibility of tight control of the
conformation recognition at the interface between the cytosine-derivatized
PDA (PDC) polymer and complementary groups will be dominated by DNA-like
base-pairs. It follows that such base-pairs, once formed will tend to
?-stack, as is the case in native DNA. This in turn will cause structural
deformation of the polymer backbone which is expected to dependent on
the degree of complementarily. Since the PDA backbone in use is a photo-conducting
material, its conformational states are expected to influence its electronic
properties. Hence, the overall outcome of this project is expected to
demonstrate the feasibility to tune the electronic, photoconductive
properties through molecular recognition. The study of this monolayer
is carried out by various techniques; including AFM, TEM, electron diffraction,
fluorescence microscopy and GIXD (two ESRF beam-time applications dedicated
to this project have been approved).
Incorporation of proteins with preferred membrane interaction
and functions into PDA films is studied in collaboration with Prof.
R. Jelinek. It was shown that these proteins are incorporated into the
film and retain their activity. Conformational and chemical changes
in the proteins are transuded to the PDA film and induce electronic
configurational change that is manifested in color transition (cf. Fig.
4).
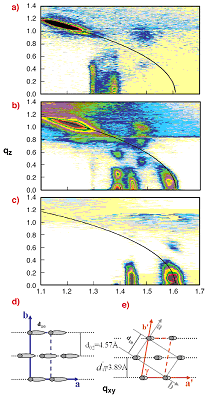
Fig. 4: GIXD maps depicting the gradual structural transformation
of PDA blue phase (a), through coexistence situation (b) to a fully
transformed ‘red’ phase (c). The black arc corresponds to
qtotal = 1.6 [Å-1]. The reconstructed unit cell of the blue phase
(d) and the red phase (e) are shown. The red phase is denser with the
alkyl chains nearly vertical.
4. Lung Surfactants
Another field of activity is related to the biomedical relevant problem
of the properties and biophysical behavior of the Lung surfactant (LS).
This material is a monolayer forming lipid that covers the lung alveoli,
reduces the surface pressure, and thus has an important role in enabling
normal breathing.
We have been studying the response of LS to in-utero contamination from
meconium. This study involved the comparison between the natural LS
replacement and a model lipid, in the presence and absence of the contamination
agent. The study has been expanded to include the dynamical behavior
of LS upon continuous compression/expansion cycles, which serve to imitate
breathing. It is observed that LS films undergo ordered organization
upon over compression, unlike typical lipid monolayers that lack the
LS protein fraction. This information may be related to different lung
functional deficiencies.

